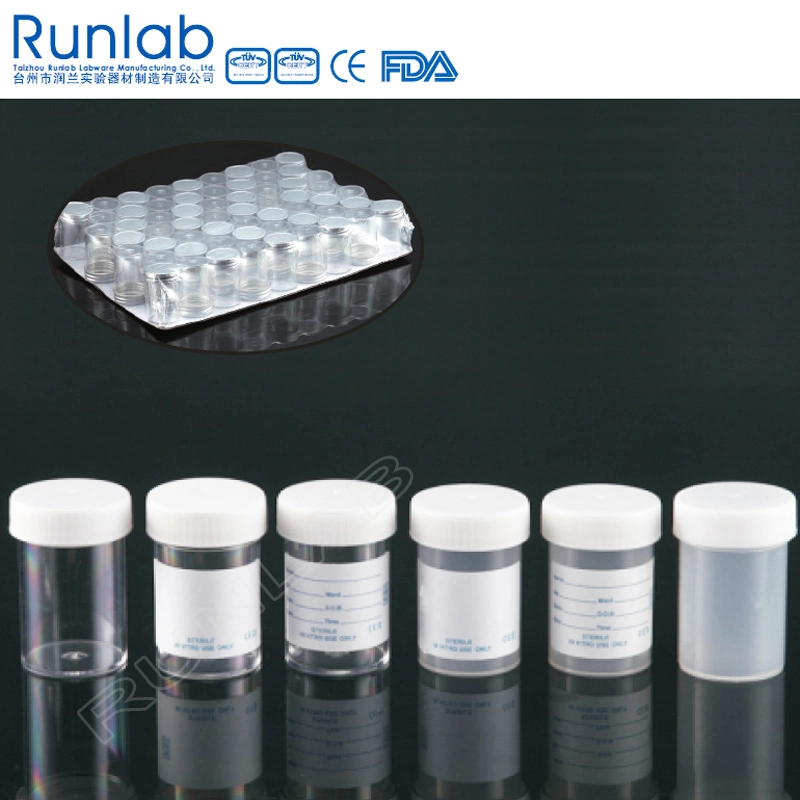
PRODUCT DESCRIPTION:
Specimen containers, 60ml, universal, screw cap with series labels
1. Manufactured from virgin polystyrene and polypropylene. All materials are non-cytotoxic
2. CE marked in accordance with the European Directive 98/79/EC
3. For In Vitro Use Only.
4. Leakproof-testing in accordance with BS5213:1975 (1989) Appendix A.
5. Unique leak tight cap ensures exceptioinal leak-proof seal
6. Containers are available in a full rane of label options (for the clinical market)
7. Package:
PRODUCT DETAILED PHOTOS:
(See above photos)

MORE DETAILS OF RUNLAB FACTORY
A. Established Date:
Runlab was established in 2005 year based on United Kingdom National Health Service (NHS) Contract.
B. Products:
As one professional manufacturer, Runlab focuses on 'laboratory plastic consumables' which are used in the clinical, research, hospital and special markets.
C. Runlab's facility:
1. covers about 20000 Sq. meters (215,000 Sq. Ft)
2. 200 workers (R&D Dept.: 5; QC Dept.:5)
3. Injection machines: 45 sets; Blowing machines: 14 sets
4. Professional laboratory for QC Detp.
5. Main markets: USA (30%) ; EUROPE (UK) (30%) ; Japan (12%) ...
6. Sales revenue: 9 million USD
D. Runlab's order systerm info.:
1. MOQ: USD5000 per order
2. Payment: TT (30% / 70%)
3. Lead-time: In general, 4 weeks for FCL shippment
E. Certificates:


FAQ:
1. Containers can be "GAMMA" stelized?
A: Runlab's containers can be GAMMA stelized, but it will be a little yellow (PP material)
2. Containers can be "E-Beam" stelized?
A: Runlab can supply E-beam sterile containers
3. What's testing standard for containers in leakage-proof feature?
A: BS5213:1975 (1989)
Specimen containers, 60ml, universal, screw cap with series labels
1. Manufactured from virgin polystyrene and polypropylene. All materials are non-cytotoxic
2. CE marked in accordance with the European Directive 98/79/EC
3. For In Vitro Use Only.
4. Leakproof-testing in accordance with BS5213:1975 (1989) Appendix A.
5. Unique leak tight cap ensures exceptioinal leak-proof seal
6. Containers are available in a full rane of label options (for the clinical market)
7. Package:
| Cat. No. | Descriptioin | Material/Cap | Pack | Unit | Case of | Sterility |
| 22203 | No label | PS / PE | Tray | 60/tray | 300 | EO |
| 22204 | Printed label | PS / PE | Tray | 60/tray | 300 | EO |
| 22205 | Plain label | PS / PE | Tray | 60/tray | 300 | EO |
| 22211 | No label | PP / PE | Tray | 60/tray | 300 | EO |
| 22212 | Printed label | PP / PE | Tray | 60/tray | 300 | EO |
| 22213 | Plain label | PP / PE | Tray | 60/tray | 300 | EO |
PRODUCT DETAILED PHOTOS:
(See above photos)

MORE DETAILS OF RUNLAB FACTORY
A. Established Date:
Runlab was established in 2005 year based on United Kingdom National Health Service (NHS) Contract.
B. Products:
As one professional manufacturer, Runlab focuses on 'laboratory plastic consumables' which are used in the clinical, research, hospital and special markets.
C. Runlab's facility:
1. covers about 20000 Sq. meters (215,000 Sq. Ft)
2. 200 workers (R&D Dept.: 5; QC Dept.:5)
3. Injection machines: 45 sets; Blowing machines: 14 sets
4. Professional laboratory for QC Detp.
5. Main markets: USA (30%) ; EUROPE (UK) (30%) ; Japan (12%) ...
6. Sales revenue: 9 million USD
D. Runlab's order systerm info.:
1. MOQ: USD5000 per order
2. Payment: TT (30% / 70%)
3. Lead-time: In general, 4 weeks for FCL shippment
E. Certificates:


FAQ:
1. Containers can be "GAMMA" stelized?
A: Runlab's containers can be GAMMA stelized, but it will be a little yellow (PP material)
2. Containers can be "E-Beam" stelized?
A: Runlab can supply E-beam sterile containers
3. What's testing standard for containers in leakage-proof feature?
A: BS5213:1975 (1989)